Keywords
Abstract
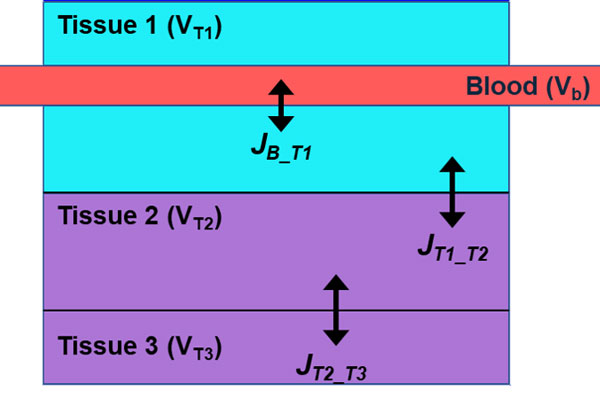
Ocular barriers to drug transport make delivery of effective doses to posterior targets exceptionally difficult. Animal models have commonly been used to evaluate drug distribution and penetrability, but translational tools to determine human dosing are lacking. Here we present a framework for modeling interspecies variation by simulating oxygen distribution in the posterior eye, from outer vitreous to the sclera. Posterior eye models of mouse, rabbit, and human are presented with modifications based solely on species-dependent anatomical and physiological differences. The model includes tissue and vascular contributions to transport. In addition to oxygen, nitric oxide and its impact on oxygen metabolism is simulated. Depth-dependent retinal oxygen partial pressure profiles are in good agreement with experimental data for all three species. The model can be further extended to evaluate the variations of retinal oxygenation in response to various drugs, formulations, administration protocols, and treatment plans. Further, this framework of ocular physiologically based pharmacokinetic/pharmacodynamic models could support animal to human translation, a critical step in the drug development process.
References
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012 May;96(5):614-8. Epub 2011 Dec 1. PMID: 22133988. https://doi.org/10.1136/bjophthalmol-2011-300539
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017 Dec;5(12):e1221-e1234. Epub 2017 Oct 11. PMID: 29032195. https://doi.org/10.1016/S2214-109X(17)30393-5
Urtti A, Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol. 1993 May-Jun;37(6):435-56. PMID: 8100087. https://doi.org/10.1016/0039-6257(93)90141-s
Agrahari V, Mandal A, Agrahari V, Trinh HM, Joseph M, Ray A, Hadji H, Mitra R, Pal D, Mitra AK. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016 Dec;6(6):735-754. PMID: 27798766; PMCID: PMC5319401. https://doi.org/10.1007/s13346-016-0339-2
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010 Sep;12(3):348-60. Epub 2010 May 1. PMID: 20437123; PMCID: PMC2895432. https://doi.org/10.1208/s12248-010-9183-3
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: An overview. World J Pharmacol. 2013;2(2):47-64. PMID: 25590022; PMCID: PMC4289909. https://doi.org/10.5497/wjp.v2.i2.47
Ali J, Fazil M, Qumbar M, Khan N, Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv. 2016;23(3):710-26. Epub 2014 Jun 3. PMID: 24892625. https://doi.org/10.3109/10717544.2014.923065
Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine (Lond). 2017 Mar;12(6):683-702. Epub 2017 Feb 10. PMID: 28186436. https://doi.org/10.2217/nnm-2016-0379
Evangelho K, Mastronardi CA, de-la-Torre A. Experimental Models of Glaucoma: A Powerful Translational Tool for the Future Development of New Therapies for Glaucoma in Humans-A Review of the Literature. Medicina (Kaunas). 2019 Jun 17;55(6):280. PMID: 31212881; PMCID: PMC6630440. https://doi.org/10.3390/medicina55060280
Krebs MP, Collin GB, Hicks WL, Yu M, Charette JR, Shi LY, Wang J, Naggert JK, Peachey NS, Nishina PM. Mouse models of human ocular disease for translational research. PLoS One. 2017 Aug 31;12(8):e0183837. PMID: 28859131; PMCID: PMC5578669. https://doi.org/10.1371/journal.pone.0183837
Mustari MJ. Nonhuman Primate Studies to Advance Vision Science and Prevent Blindness. ILAR J. 2017 Dec 1;58(2):216-225. PMID: 28575309; PMCID: PMC5886335. https://doi.org/10.1093/ilar/ilx009
Carvalho C, Gaspar A, Knight A, Vicente L. Ethical and Scientific Pitfalls Concerning Laboratory Research with Non-Human Primates, and Possible Solutions. Animals (Basel). 2018 Dec 29;9(1):12. PMID: 30597951; PMCID: PMC6356609. https://doi.org/10.3390/ani9010012
Manning FJ, Bond EC, Berns KI. The Washington Regional Primate Research Center. In: Resource Sharing in Biomedical Research. National Academies Press; 1996.
Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp Eye Res. 2015 Aug;137:111-24. Epub 2015 May 12. Erratum in: Exp Eye Res. 2018 Jan 31;169:60. PMID: 25975234. https://doi.org/10.1016/j.exer.2015.05.003
Ahmed I, Patton TF. Disposition of timolol and inulin in the rabbit eye following corneal versus non-corneal absorption. Int J Pharm. 1987 Aug 1;38(1-3):9-21.
Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004 May;78(5):917-24. PMID: 15051473. https://doi.org/10.1016/j.exer.2004.01.003
Carcaboso AM, Bramuglia GF, Chantada GL, Fandiño AC, Chiappetta DA, de Davila MT, Rubio MC, Abramson DH. Topotecan vitreous levels after periocular or intravenous delivery in rabbits: an alternative for retinoblastoma chemotherapy. Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3761-7. PMID: 17652749. https://doi.org/10.1167/iovs.06-1152
Gwon A. The Rabbit in Cataract/IOL Surgery. In: Tsonis PA, (editor). Animal Models in Eye Research. Academic Press; 2008. pp. 184–204. https://doi.org/10.1016/B978-0-12-374169-1.00013-8
Akhtar A. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics. 2015 Oct;24(4):407-19. PMID: 26364776; PMCID: PMC4594046. https://doi.org/10.1017/S0963180115000079
Gawrylewski A. The Trouble with Animal Models. (2007) The Scientist Magazine. Available from: https://www.the-scientist.com/uncategorized/the-trouble-with-animal-models-46344
Cattaneo C, Maderna E, Rendinelli A, Gibelli D. Animal experimentation in forensic sciences: How far have we come? Forensic Sci Int. 2015 Sep;254:e29-35. Epub 2015 Jul 15. PMID: 26216717. https://doi.org/10.1016/j.forsciint.2015.06.024
Cohen L. Relationships between visual function and metabolism. Biochem Eye. 1965:36-50.
Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99-116. Epub 2010 Jul 22. PMID: 23226947; PMCID: PMC3515641. https://doi.org/10.2147/EB.S9078
Anderson B, Saltzman HA. Retinal oxygen utilization measured by hyperbaric blackout. Arch Ophthalmol. 1964 Dec 1;72(6):792-5.
Ames A 3rd. Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol. 1992;70 Suppl:S158-64. PMID: 1295666. https://doi.org/10.1139/y92-257
One Hour Optical. Retinal Disease, Problems, Treatments. What are Retinal Diseases? Available from: https://onehouroptical.com/eye-health/what-are-retinal-diseases
Najmanová E, Pluháček F, Botek M, Krejčí J, Jarošová J. Intraocular Pressure Response to Short- Term Extreme Normobaric Hypoxia Exposure. Front Endocrinol (Lausanne). 2019 Jan 7;9:785. PMID: 30666235; PMCID: PMC6330315. https://doi.org/10.3389/fendo.2018.00785
Wójcik-Gryciuk A, Skup M, Waleszczyk WJ. Glaucoma -state of the art and perspectives on treatment. Restor Neurol Neurosci. 2016;34(1):107-23. PMID: 26684267; PMCID: PMC4927811. https://doi.org/10.3233/RNN-150599
Acharya R, Ng YE, Suri JS. Image modeling of the human eye. Artech House; 2008.
Gajraj R. A study of drug transport in the vitreous humor: effect of drug size; comparing micro-and macro-scale diffusion; assessing vitreous models; and obtaining in vivo data (Doctoral dissertation. 2012).
Ferguson LR, Dominguez JM 2nd, Balaiya S, Grover S, Chalam KV. Retinal Thickness Normative Data in Wild-Type Mice Using Customized Miniature SD-OCT. PLoS One. 2013 Jun 27;8(6):e67265. PMID: 23826252; PMCID: PMC3695045. https://doi.org/10.1371/journal.pone.0067265
Faude F, Francke M, Makarov F, Schuck J, Gärtner U, Reichelt W, Wiedemann P, Wolburg H, Reichenbach A. Experimental retinal detachment causes widespread and multilayered degeneration in rabbit retina. J Neurocytol. 2001 May;30(5):379-90. PMID: 11951049. https://doi.org/10.1023/a:1015061525353
van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Abràmoff MD. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3660-5. Epub 2010 Feb 3. PMID: 20130282; PMCID: PMC2904016. https://doi.org/10.1167/iovs.09-5041
Manning PJ. The Biology of the Laboratory Rabbit. Academic Press; 2014.
Ozkaya A, Alkin Z, Karakucuk Y, Karatas G, Fazil K, Gurkan Erdogan M, Perente I, Taskapili M. Thickness of the retinal photoreceptor outer segment layer in healthy volunteers and in patients with diabetes mellitus without retinopathy, diabetic retinopathy, or diabetic macular edema. Saudi J Ophthalmol. 2017 Apr-Jun;31(2):69-75. Epub 2017 Jan 4. PMID: 28559716; PMCID: PMC5436385. https://doi.org/10.1016/j.sjopt.2016.12.006
Terauchi G, Shinoda K, Matsumoto CS, Watanabe E, Matsumoto H, Mizota A. Recovery of photoreceptor inner and outer segment layer thickness after reattachment of rhegmatogenous retinal detachment. Br J Ophthalmol. 2015 Oct;99(10):1323-7. Epub 2015 Apr 3. PMID: 25841234. https://doi.org/10.1136/bjophthalmol-2014-306252
Mac Gabhann F, Demetriades AM, Deering T, Packer JD, Shah SM, Duh E, Campochiaro PA, Popel AS. Protein transport to choroid and retina following periocular injection: theoretical and experimental study. Ann Biomed Eng. 2007 Apr;35(4):615-30. Epub 2007 Feb 3. PMID: 17277991. https://doi.org/10.1007/s10439-006-9238-x
Karawya S, Said DG, Salaheldin MM, Zaky I. Impact of Intravitreal Injection of Bevacizumab (Avastin) on Rabbit’s Choroid and Retina. Middle East Afr J Ophthalmol. 2008 Apr;15(2):67-72. PMID: 21346840; PMCID: PMC3038111. https://doi.org/10.4103/0974-9233.51995
Ko F, Foster PJ, Strouthidis NG, Shweikh Y, Yang Q, Reisman CA, Muthy ZA, Chakravarthy U, Lotery AJ, Keane PA, Tufail A, Grossi CM, Patel PJ; UK Biobank Eye & Vision Consortium. Associations with Retinal Pigment Epithelium Thickness Measures in a Large Cohort: Results from the UK Biobank. Ophthalmology. 2017 Jan;124(1):105-117. Epub 2016 Oct 6. PMID: 27720551. https://doi.org/10.1016/j.ophtha.2016.07.033
Prince JH, Diesem CD, Eglitis I, Ruskell GL. Anatomy and histology of the eye and orbit in domestic animals. 1960.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009 May;147(5):811-5. Epub 2009 Feb 20. PMID: 19232559. https://doi.org/10.1016/j.ajo.2008.12.008
Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010 Sep;150(3):325-329.e1. Epub 2010 Jun 29. PMID: 20591395; PMCID: PMC2926223. https://doi.org/10.1016/j.ajo.2010.04.018
Gargini C, Novelli E, Piano I, Biagioni M, Strettoi E. Pattern of retinal morphological and functional decay in a light-inducible, rhodopsin mutant mouse. Sci Rep. 2017 Jul 18;7(1):5730. PMID: 28720880; PMCID: PMC5516022. https://doi.org/10.1038/s41598-017-06045-x
Reichenbach A, Ziegert M, Schnitzer J, Pritz-Hohmeier S, Schaaf P, Schober W, Schneider H. Development of the rabbit retina. V. The question of ‘columnar units’. Brain Res Dev Brain Res. 1994 May 13;79(1):72-84. PMID: 8070066. https://doi.org/10.1016/0165-3806(94)90050-7
Michels RG, Wilkinson CP, Rice TA. Retinal detachment. Mosby: St. Louis; 1990.
Nagra M, Gilmartin B, Thai NJ, Logan NS. Determination of retinal surface area. J Anat. 2017 Sep;231(3):319-324. Epub 2017 Jun 16. PMID: 28620965; PMCID: PMC5554828. https://doi.org/10.1111/joa.12641
Panda-Jonas S, Jonas JB, Jakobczyk M, Schneider U. Retinal photoreceptor count, retinal surface area, and optic disc size in normal human eyes. Ophthalmology. 1994 Mar;101(3):519-23. PMID: 8127572. https://doi.org/10.1016/s0161-6420(94)31305-4
Duong TQ, Pardue MT, Thulé PM, Olson DE, Cheng H, Nair G, Li Y, Kim M, Zhang X, Shen Q. Layer-specific anatomical, physiological and functional MRI of the retina. NMR Biomed. 2008 Nov;21(9):978-96. PMID: 18792422; PMCID: PMC2752861. https://doi.org/10.1002/nbm.1311
Hyvdrinen L. Vascular Structures of the Rabbit Retina. Acta Ophthalmol (Copenh.) 1967;45:852–861.
Muir ER, Rentería RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012 Sep 21;53(10):6488-94. PMID: 22915034; PMCID: PMC4045095. https://doi.org/10.1167/iovs.12-9758
Nilsson S, Alm, A. Determination of ocular blood flows with the microsphere method. In: Ocular Blood Flow. Springer; 2012. pp. 25–47.
Sebag J, Tang M, Brown S, Sadun AA, Charles MA. Effects of pentoxifylline on choroidal blood flow in nonproliferative diabetic retinopathy. Angiology. 1994 Jun;45(6):429-33. PMID: 8203768. https://doi.org/10.1177/000331979404500603
Feke GT, Tagawa H, Deupree DM, Goger DG, Sebag J, Weiter JJ. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):58-65. PMID: 2643588.
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep. 2017 Feb 10;7:42201. PMID: 28186181; PMCID: PMC5301488. https://doi.org/10.1038/srep42201
Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N, Beaudoin F, Leboeuf D, De Guire V, Binet F, Dejda A, Rezende FA, Miloudi K, Sapieha P. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013 Oct 1;18(4):505-18. PMID: 24093675. https://doi.org/10.1016/j.cmet.2013.09.003
Sugano Y, Sekiryu T, Furuta M, Tomita R, Shintake H, Maehara H, Ojima A. Morphometrical evaluation of the choriocapillaris imaged by swept-source optical coherence tomography angiography. Clin Ophthalmol. 2018 Nov 5;12:2267-2276. PMID: 30464388; PMCID: PMC6223397. https://doi.org/10.2147/OPTH.S179634
Goto S, Onishi A, Misaki K, Yonemura S, Sugita S, Ito H, Ohigashi Y, Ema M, Sakaguchi H, Nishida K, Takahashi M. Neural retina-specific Aldh1a1 controls dorsal choroidal vascular development via Sox9 expression in retinal pigment epithelial cells. Elife. 2018 Apr 3;7:e32358. PMID: 29609731; PMCID: PMC5882243. https://doi.org/10.7554/eLife.32358
Zhu Q, Xing X, Zhu M, Xiao H, Ma L, Chen L, Liang J, Yuan Y, Song E. A New Approach for the Segmentation of Three Distinct Retinal Capillary Plexuses Using Optical Coherence Tomography Angiography. Transl Vis Sci Technol. 2019 Jun 28;8(3):57. PMID: 31293812; PMCID: PMC6602150. https://doi.org/10.1167/tvst.8.3.57
Kim TH, Son T, Lu Y, Alam M, Yao X. Comparative Optical Coherence Tomography Angiography of Wild-Type and rd10 Mouse Retinas. Transl Vis Sci Technol. 2018 Dec 28;7(6):42. PMID: 30619662; PMCID: PMC6314228. https://doi.org/10.1167/tvst.7.6.42
Condren AB, Kumar A, Mettu P, Liang KJ, Zhao L, Tsai JY, Fariss RN, Wong WT. Perivascular mural cells of the mouse choroid demonstrate morphological diversity that is correlated to vasoregulatory function. PLoS One. 2013;8(1):e53386. Epub 2013 Jan 4. PMID: 23308209; PMCID: PMC3537675. https://doi.org/10.1371/journal.pone.0053386
Bhutto IA, Amemiya T. Microvascular architecture of the rat choroid: corrosion cast study. Anat Rec. 2001 Sep 1;264(1):63-71. PMID: 11505372. https://doi.org/10.1002/ar.1102
Lutty GA, Hasegawa T, Baba T, Grebe R, Bhutto I, McLeod DS. Development of the human choriocapillaris. Eye (Lond). 2010 Mar;24(3):408-15. Epub 2010 Jan 15. PMID: 20075975; PMCID: PMC4848024. https://doi.org/10.1038/eye.2009.318
Fryczkowski AW. Anatomical and functional choroidal lobuli. Int Ophthalmol. 1994;18(3):131-41. PMID: 7852018. https://doi.org/10.1007/BF00915961
McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4982-91. Epub 2009 Apr 8. PMID: 19357355; PMCID: PMC4829357. https://doi.org/10.1167/iovs.09-3639
Ninomiya H, Inomata T. Microvasculature of the mouse eye: scanning electron microscopy of vascular corrosion casts. J Exp Anim Sci. 2006 Dec 12;43(3):149-59.
Chandrasekera E, An D, McAllister IL, Yu DY, Balaratnasingam C. Three-Dimensional Microscopy Demonstrates Series and Parallel Organization of Human Peripapillary Capillary Plexuses. Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4327-4344. PMID: 30193305. https://doi.org/10.1167/iovs.18-24105
Goldman, D. Simulations of Capillary Network Oxygen Transport During Transient Ischemia in the Presence and Absence of Tissue Myoglobin. In: Wilson DF, Evans SM, Biaglow J, Pastuszko A (editors). Oxygen Transport To Tissue XXIII: Oxygen Measurements in the 21st Century: Basic Techniques and Clinical Relevance. Springer; 2003.pp. 355–359. https://doi.org/10.1007/978-1-4615-0205-0_58
Goldman D, Popel AS. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J Theor Biol. 2000 Sep 21;206(2):181-94. PMID: 10966756. https://doi.org/10.1006/jtbi.2000.2113
Goldman D, Popel AS. A computational study of the effect of vasomotion on oxygen transport from capillary networks. J Theor Biol. 2001 Mar 21;209(2):189-99. PMID: 11401461. https://doi.org/10.1006/jtbi.2000.2254
Goldman D, Popel AS. Computational Modeling of Oxygen Transport from Complex Capillary Networks. In: Eke A, Delpy DT (editors). Oxygen Transport to Tissue XXI (eds.) Springer; 1999. pp. 555–563. https://doi.org/10.1007/978-1-4615-4717-4_65
Avtar R, Tandon D. Mathematical modelling of intraretinal oxygen partial pressure. Trop J Pharm Res. 2008 Dec 11;7(4):1107-16.
Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003 Apr;121(4):547-57. PMID: 12695252. https://doi.org/10.1001/archopht.121.4.547
Causin P, Guidoboni G, Malgaroli F, Sacco R, Harris A. Blood flow mechanics and oxygen transport and delivery in the retinal microcirculation: multiscale mathematical modeling and numerical simulation. Biomech Model Mechanobiol. 2016 Jun;15(3):525-42. Epub 2015 Aug 1. PMID: 26232093. https://doi.org/10.1007/s10237-015-0708-7
Lamkin-Kennard KA, Buerk DG, Jaron D. Interactions between NO and O2 in the microcirculation: a mathematical analysis. Microvasc Res. 2004 Jul;68(1):38-50. PMID: 15219419. https://doi.org/10.1016/j.mvr.2004.03.001
Yu DY, Cringle SJ, Su EN. Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci. 2005 Dec;46(12):4728-33. PMID: 16303972. https://doi.org/10.1167/iovs.05-0694
Yu DY, Cringle SJ. Oxygen distribution in the mouse retina. Invest Ophthalmol Vis Sci. 2006 Mar;47(3):1109-12. PMID: 16505048. https://doi.org/10.1167/iovs.05-1118
Yu DY, Cringle SJ. Oxygen distribution in the mouse retina. Invest Ophthalmol Vis Sci. 2006 Mar;47(3):1109-12. PMID: 16505048. https://doi.org/10.1167/iovs.05-1118
Przekwas A, Friend T, Teixeira R, Chen ZJ, Wilkerson P. Spatial modeling tools for cell biology. CFD RESEARCH CORP HUNTSVILLE AL; 2006 Oct 1.
Roh H-D, Goldstick TK, Linsenmeier RA. Spatial variation of the local tissue oxygen diffusion coefficient measured in situ in the cat retina and cornea. In: Oxygen Transport to Tissue XII. Springer: 1990. pp. 127–136.
Goldstick TK, Ciuryla VT, Zuckerman L. Diffusion of oxygen in plasma and blood. Adv Exp Med Biol. 1976;75:183–190.
Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1076-82. 1993.1735. PMID: 8323533. https://doi.org/10.1006/bbrc
Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol. 1992 Feb;99(2):177-97. PMID: 1613482; PMCID: PMC2216610. https://doi.org/10.1085/jgp.99.2.177
Billett HH. Hemoglobin and Hematocrit. In: Walker HK, Hall WD, Hurst JW (editors). Clinical Methods: The History, Physical, and Laboratory Examinations. Butterworths; 1990.
Hedrich H. The laboratory mouse. Academic Press; 2004.
Black DM, Gilardi KV, Hamilton LP, Williams E, Williams DF, Kelly PA, Gardner I. Hematologic and biochemistry reference values for the endangered riparian brush rabbit (Sylvilagus bachmani riparius). J Wildl Dis. 2009 Apr;45(2):491-6. PMID: 19395758. https://doi.org/10.7589/0090-3558-45.2.491
Quaknine-Orlando B, Samama CM, Riou B, Bonnin P, Guillosson JJ, Beaumont JL, Coriat P. Role of the hematocrit in a rabbit model of arterial thrombosis and bleeding. Anesthesiology. 1999 May;90(5):1454-61. PMID: 10319795. https://doi.org/10.1097/00000542-199905000-00031
Moore JA, Ethier CR. Oxygen mass transfer calculations in large arteries. J Biomech Eng. 1997 Nov;119(4):469-75. PMID: 9407287. https://doi.org/10.1115/1.2798295
Shimizu S, Enoki Y, Kohzuki H, Ohga Y, Sakata S. Determination of Hüfner’s factor and inactive hemoglobins in human, canine, and murine blood. Jpn J Physiol. 1986;36(5):1047-51. PMID: 3560533. https://doi.org/10.2170/jjphysiol.36.1047
Palkovits S, Told R, Schmidl D, Boltz A, Napora KJ, Lasta M, Kaya S, Werkmeister RM, Popa-Cherecheanu A, Garhöfer G, Schmetterer L. Regulation of retinal oxygen metabolism in humans during graded hypoxia. Am J Physiol Heart Circ Physiol. 2014 Nov 15;307(10):H1412-8. Epub 2014 Sep 12. PMID: 25217648. https://doi.org/10.1152/ajpheart.00479.2014
Blair NP, Wanek J, Felder AE, Brewer KC, Joslin CE, Shahidi M. Inner Retinal Oxygen Delivery, Metabolism, and Extraction Fraction in Ins2Akita Diabetic Mice. Invest Ophthalmol Vis Sci. 2016 Nov 1;57(14):5903-5909. PMID: 27802520; PMCID: PMC5096417. https://doi.org/10.1167/iovs.16-20082
Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Prog Retin Eye Res. 2017 May;58:115-151. Epub 2017 Jan 18. PMID: 28109737; PMCID: PMC5441959. https://doi.org/10.1016/j.preteyeres.2017.01.003
Sobecki R. [Studies on oxygen content in aqueous humor of the anterior chamber of the rabbit’s eye--I. Oxygen content in aqueous humor of anterior chamber and arterial blood under physiologic conditions]. Klin Oczna 1993;95161–162.
Carlsen E, Comroe JH. The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. J Gen Physiol. 1958;42:83–107.
Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998 Jun;274(6):H2163-76. PMID: 9841542. https://doi.org/10.1152/ajpheart.1998.274.6.H2163
Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998 May;274(5):H1705-14. PMID: 9612383. https://doi.org/10.1152/ajpheart.1998.274.5.H1705
Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):355-60. PMID: 11134509; PMCID: PMC14594. https://doi.org/10.1073/pnas.011379598
Gray LH, Steadman JM. Determination of the oxyhaemoglobin dissociation curves for mouse and rat blood. J Physiol. 1964;175:161–171.
Jelkmann W, Bauer C. Oxygen affinity and phosphate compounds of red blood cells during intrauterine development of rabbits. Pflügers Archiv. 1977;372:149–156.
Dalbey K, et al. Dakota A Multilevel Parallel Object-Oriented Framework for Design Optimization Parameter Estimation Uncertainty Quantification and Sensitivity Analysis: Version 6.12 Theory Manual. Available from: https://www.osti.gov/biblio/1630693 (2020). https://doi.org/10.2172/1630693
Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986 Oct;88(4):521-42. PMID: 3783124; PMCID: PMC2228847. https://doi.org/10.1085/jgp.88.4.521
Kaufman PL, Levin LA, Adler FH, Alm A. Adler’s Physiology of the Eye. Elsevier Health Sciences; 2011.
Harris A, Kagemann L, Cioffi GA. Assessment of human ocular hemodynamics. Surv Ophthalmol. 1998 May-Jun;42(6):509-33. PMID: 9635901. https://doi.org/10.1016/s0039-6257(98)00011-3
Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009 Sep;21(2):92-103. Epub 2009 Jul 12. PMID: 19602444; PMCID: PMC2779337. https://doi.org/10.1016/j.niox.2009.07.002
Roberts PA, Gaffney EA, Luthert PJ, Foss AJE, Byrne HM. Mathematical and computational models of the retina in health, development and disease. Prog Retin Eye Res. 2016 Jul;53:48-69. Epub 2016 Apr 7. PMID: 27063291. https://doi.org/10.1016/j.preteyeres.2016.04.001
Scott A, Fruttiger M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond). 2010 Mar;24(3):416-21. Epub 2009 Dec 11. PMID: 20010791. https://doi.org/10.1038/eye.2009.306
Fu Y, Dong Y, Gao Q. Age-related cataract and macular degeneration: Oxygen receptor dysfunction diseases. Med Hypotheses. 2015 Sep;85(3):272-5. Epub 2015 Jun 1. PMID: 26049822. https://doi.org/10.1016/j.mehy.2015.05.020
Thomas DD. Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide. Redox Biol. 2015 Aug;5:225-233. Epub 2015 May 22. PMID: 26056766; PMCID: PMC4473092. https://doi.org/10.1016/j.redox.2015.05.002
German C, Pilvankar M, Przekwas A. Computational framework for predictive PBPK-PD-Tox simulations of opioids and antidotes. J Pharmacokinet Pharmacodyn. 2019 Dec;46(6):513-529. Epub 2019 Aug 8. PMID: 31396799. https://doi.org/10.1007/s10928-019-09648-1