Keywords
Abstract
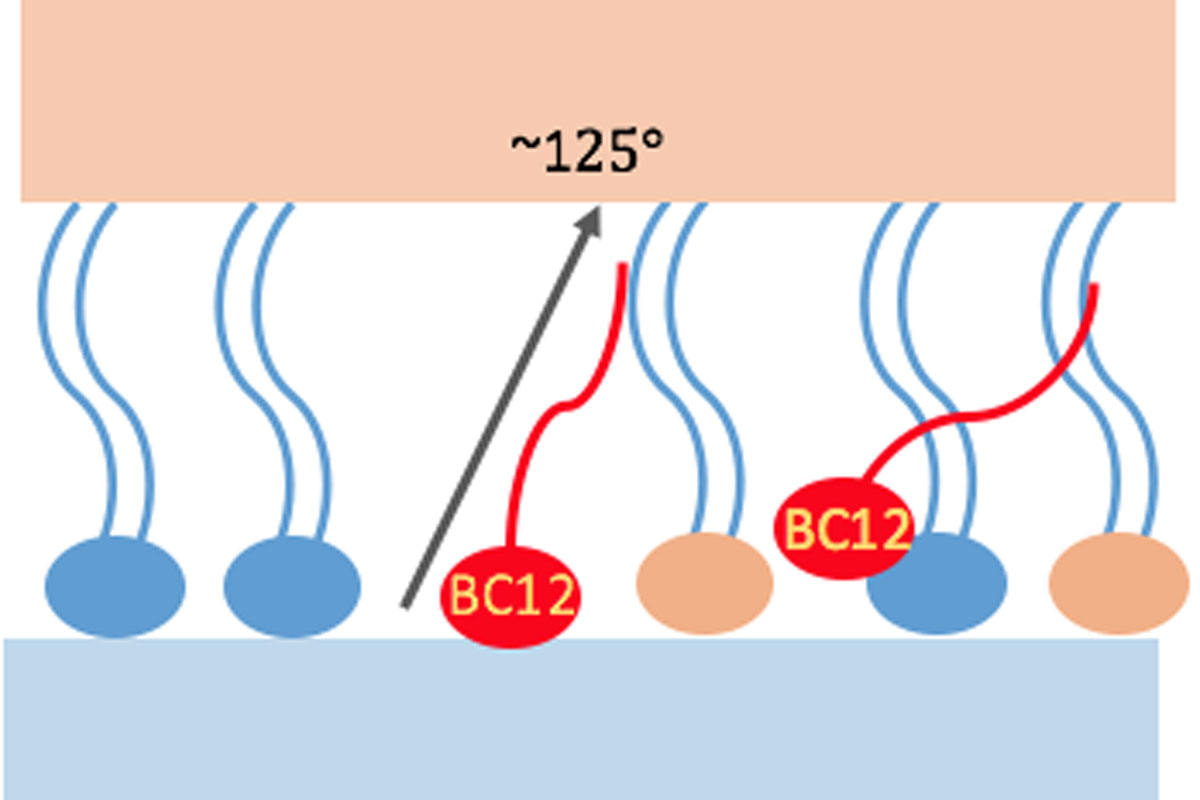
Benzalkonium chloride (BAK) is a mixture of aliphatic C12 and C14 quaternary ammoniums. These molecules are traditionally used to preserve eye drops because of their bactericidal and bacteriostatic properties. The compounds of BAK have an amphiphilic character, hence it can be assumed that on the ocular surface they can interact and alter the properties of the tear film lipid layer (TFLL). Indeed, BAK was demonstrated to decrease the breakup time in patients, which is a hallmark of TFLL destabilization. The amphiphilic and water-soluble C12 and C14 BAK molecules are expected to act predominantly at the aqueous-lipid interface that, as we have demonstrated earlier, is populated mostly by polar lipids.7,8 Notably, these BAK species are short-chain analogues of cetalkonium chloride (CKC) that, as we have shown previously, interact with the TFLL model, improving its stability. We hypothesize that by influencing polar lipids, BAK (C12 and C14) can alter the details of molecular-level interactions between individual species of TFLL and indirectly influence the macroscopic behavior of the film, in particular its organization and stability.
References
Kurup TR, Wan L, Chan L. Preservative requirements in emulsions. Pharm Acta Helv. 1992;67(7):204-208.
Campanac C, Pineau L, Payard A, Baziard-Mouysset G, Roques C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother. 2002;46(5):1469-1474. doi: 10.1128/AAC.46.5.1469-1474.2002.
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312-334. doi: 10.1016/j.preteyeres.2010.03.001.
Wilson W, Duncan A, Jay J. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br J Ophthalmol. 1975;59(11):667-669. doi: 10.1136/bjo.59.11.667.
Campagna P, Macri A, Rolando M, Calabria G. Chronic topical eye preservative‐free beta‐blocker therapy effect on the ocular surface in glaucomatous patients. Acta Ophthalmol Scand Suppl. 1997;75(S224):53. doi: 10.1111/j.1600-0420.1997.tb00480.x
Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99(7):1082-1088. doi: 10.1016/S0161-6420(92)31847-0.
Wizert A, Iskander DR, Cwiklik L. Organization of lipids in the tear film: a molecular-level view. PLoS One. 2014;9(3). doi: 10.1371/journal.pone.0092461. PubMed PMID: WOS:000333352800111.
Cwiklik L. Tear film lipid layer: A molecular level view. Biochim Biophys Acta. 2016;(10):2421-2430. doi: 10.1016/j.bbamem.2016.02.020.
Nencheva Y, Olzynska A, Melcrova A, et al. Improving stability of tear film lipid layer via concerted action of two drug molecules: a biophysical view. Biophys J. 2018;114(3):104a. doi: 10.1016/j.bpj.2017.11.609.
Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. The MARTINI force field: coarse grained model for biomolecular simulations. J Phys Chem B. 2007;111(27):7812-7824. doi: 10.1021/jp071097f. PubMed PMID: 17569554.
Olżyńska A, Cwiklik L. Behavior of sphingomyelin and ceramide in a tear film lipid layer model. Ann Anat. 2017;210:128-134. doi: 10.1016/j.aanat.2016.10.005.
Wizert A, Iskander DR, Cwiklik L. Interaction of lysozyme with a tear film lipid layer model: A molecular dynamics simulation study. Biochim Biophys Acta. 2017;1859(12):2289-2296. doi: 10.1016/j.bbamem.2017.08.015. PubMed PMID: WOS:000415770900002.
Rantamaki AH, Seppanen-Laakso T, Oresic M, Jauhiainen M, Holopainen JM. Human tear fluid lipidome: from composition to function. PLoS One. 2011;6(5):e19553. doi: 10.1371/journal.pone.0019553. PubMed PMID: 21573170; PubMed Central PMCID: PMC3088682.
Olzynska A, Cwiklik L. Behavior of sphingomyelin and ceramide in a tear film lipid layer model. Ann Anat. 2017;210:128-134. doi: 10.1016/j.aanat.2016.10.005. PubMed PMID: WOS:000395610600016.
Robert P-Y, Cochener B, Amrane M, et al. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol. 2016;26(6):546-555. doi: 10.5301/ejo.5000830.
Georgiev GA, Yokoi N, Nencheva Y, Peev N, Daull P. Surface chemistry interactions of cationorm with films by human meibum and tear film compounds. Int J Mol Sci. 2017;18(7):1558. doi: 10.3390/ijms18071558.
Amrane M, Creuzot-Garcher C, Robert P-Y, et al. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease–A randomised comparative study. J Fr Ophthalmol. 2014;37(8):589-598. doi: 10.1016/j.jfo.2014.05.001.
Baudouin C, de Lunardo C. Short term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82(1):39-42. doi: 10.1136/bjo.82.1.39.
Ishibashi T, Yokoi N, Kinoshita S. Comparison of the short-term effects on the human corneal surface of topical timolol maleate with and without benzalkonium chloride. J Glaucoma. 2003;12(6):486-90.